UCSD Researchers Publish Findings on Evolution, Cell Biology in PNAS
San Diego, CA, November 7, 2006 -- A new model for protein kinase activation. A gene in mammals that could one day be targeted to the nuclear pore. Those are two of the findings by Calit2-affiliated researchers at UCSD, published in the Nov. 6-10 online early edition of the Proceedings of the National Academy of Sciences (PNAS).
|
Faculty and graduate students from UCSD's Bioengineering, Chemistry and Biochemistry, Biology and Pharmacology departments as well as the San Diego Supercomputer Center (SDSC) and Scripps Institution of Oceanography co-authored papers in the latest PNAS. Using protein structures, Scripps graduate student Chris Dupont, Protein Data Bank director Philip Bourne and their colleagues found that trace-metal usage by present-day organisms probably derives from major changes in ocean chemistry, such as those stemming from a rise in atmospheric oxygen more than two billion years ago. [For details, read the Nov. 5 news release .]
Other UCSD papers include two in the Cell Biology section of PNAS.
Protein Kinase Activation
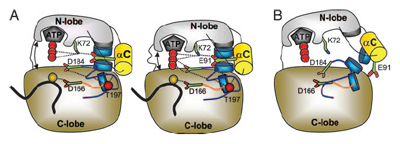
A team led by Howard Hughes Medical Institute investigator Susan Taylor from the Chemistry and Biochemistry department at UCSD, and Lynn TenEyck from SDSC, studied a set of protein kinases that were crystallized in active and inactive states.
|
In previous studies, researchers analyzed the kinases' tertiary structures. Instead, the UCSD scientists compared the surfaces of these molecules using a recently developed surface matching method based on the edge comparison and combination algorithm. "We have detected a set of 30 residues whose positions on the protein surface were highly conserved for different serine-threonine and tyrosine kinases," report the authors, noting that while some of the residues were known to be functionally important, others were not-until now. "We have analyzed these residues and their possible roles in the activation process."
Subsequent to that analysis, the authors developed a general model of protein kinase activation in which the most important feature of the activation is a 'spine' formation that is dynamically assembled in all active kinases. The spine spans the molecule and plays a coordinating role in activated kinases. Conversely, in inactive kinases, the spine is disordered-explaining "how stabilization of the whole molecule is achieved upon phosphorylation." Phosphorylation is the addition of a phosphate group to a protein or small molecule, and many enzymes and receptors are turned on by the process, making is one of the most important regulatory events in eukaryotes, such as animals and plants. Explain the researchers: "This model explains how the presence of a phosphate group in the activation loop determines the position of the catalytically important aspartate in the Asp-Phe-Gly motif."
Understanding the ELYS Gene
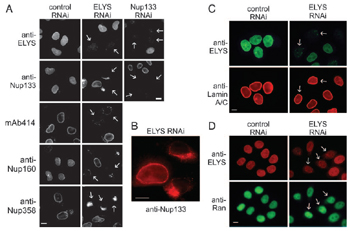
Another paper in PNAS is by lead author, graduate student Beth Rasala, senior author Douglass Forbes, and UCSD biology professor Steven Briggs. Briggs was recently appointed co-director of Calit2's new Center for Algorithmic and Systems Biology (CASB), which will be inaugurated November 30 during the Algorithmic Biology 2006 conference in Atkinson Hall.
|
"We have identified an unexpected member of the nuclear pore and kinetochore that functions in both pore assembly at the nucleus and faithful cell division," report the researchers. Briggs and his team's explanation for why ELYS was not identified until now? The human and mouse ELYS protein sequences were not placed in the National Center for Biotechnology Information (NCBI) database until after publication of the proteomics study. According to the authors, their "current findings in mammalian cells [show] that ELYS RNAi disrupts the nuclear pore and cell division." They also suggest that if it is a DNA-binding protein, ELYS could eventually play a role in "potential targeting of active vertebrate genes to the nuclear pore" (which until now has only been observed in yeast).
Related Links
Proceedings of the National Academy of Sciences
National Center for Biotechnology Information
Center for Algorithmic and Systems Biology
News Release: UC San Diego Scientists Establish Connection Between Life Today and Ancient Changes in Ocean Chemistry