Atomic Age Appropriate
By Anna Lynn Spitzer
Irvine, Calif., April 2009 -- Splitting a uranium atom produces 10 million times more energy than combusting a carbon atom from coal. It also produces widespread dissent.
Proponents of nuclear energy cite its “clean” footprint, long-term availability and efficiency. One ton of uranium produces more energy than several million tons of coal or several million barrels of oil, without releasing pollutants into the atmosphere.
|
UCI chemical engineering assistant professor Mikael Nilsson wants to
alleviate one of those concerns; he is researching ways to reduce and recycle nuclear waste. Chemically separating elements in spent nuclear fuel, Nilsson says, allows much of it to be reused and the remainder to be altered so it poses less of a threat.
Reducing the Risk
Nuclear energy is the result of neutrons bombarding the nuclei of uranium or plutonium atoms, which causes the atoms to split in a process called nuclear fission. Splitting the atoms releases tremendous heat, as well as additional neutrons. The heat is used to drive generators that produce electricity, while the neutrons continue to split other atoms, causing a chain reaction.
The problem, however, is that the reaction leaves behind highly radioactive waste material. The toxic detritus is stored: first in cooling tanks and then, when those become full, in lead-lined concrete buildings at the nuclear reactor sites.
Recycling plants in Europe and Japan separate the uranium and plutonium from the spent fuel and reuse it in new fuel, leaving less harmful material to accumulate. The U.S. prohibited this separation process about 25 years ago,fearing it would lead to proliferation and facilitate terrorism. Because all nuclear waste in this country is stored, stockpiles from 104 nuclear reactors in 35 states continue to grow at the rate of 2,000 tons per year, with no end in sight.
And it’s not short-term storage either. The components of spent nuclear fuel can last almost forever; Uranium-235, for example, has a half-life of 704 million years, meaning it takes that long for just half its atoms to decay.
Yucca Mountain, a national waste repository in Nevada, was scheduled for completion next year, but after 20 years of planning and $9 million in expenditures, it was recently eliminated from the federal budget.
There is an upside though. U.S. policy was amended in 2006, so scientists like Nilsson have redoubled their efforts to understand chemical separation of elements in spent fuel.
Recycling Toxic Elements
Nilsson seeks to separate out the most long-lived and toxic of the elements that remain in spent fuel after the uranium and plutonium are removed. Some of these elements then can be reconstituted into new fuel.
Transmutation, as it’s called, is not new, but the advanced separation process has never been entirely successful with nuclear fuel. “This hasn’t been done because it’s hard to find a good process,” he says. “The chemicals all suffer from poor stability, or they’re too poisonous or they may be too difficult to control in a commercial plant.”
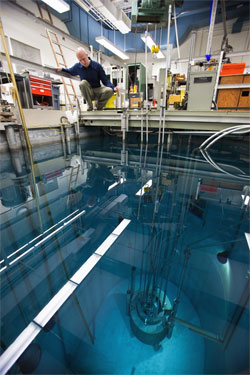
Nilsson’s approach is to simulate material in spent nuclear fuel by submerging samples of a number of different materials into UCI’s nuclear reactor in the basement of Rowland Hall. The nuclear fission process creates neutrons, which in turn can activate the material and create radioisotopes. He adds these radioactive elements to a solution that mimics nuclear fuel, allowing him to experiment with less fear of contamination.“There are so many elements in spent nuclear fuel – almost half of the periodic table – so in order to be able to separate them, you have to find a way to select just the ones you want to try to recycle,” he explains.
By tracking the radioactive elements, he learns how they behave, so he can identify them and ultimately, remove them from the “chemical soup.” “It’s easy to follow them once they’re radioactive,” he says, and adds, laughing, “The beautiful thing about working with radioactivity is that it’s so easy to detect.”
Decreasing Storage Time
The first step is to identify the materials that have the longest half-lives, because they will be there for the longest time, Nilsson says. When these elements are irradiated, their properties may change and they can become other elements. These altered compounds usually have shorter half-lives, meaning they decay faster.
“Some of it will always have to be stored somewhere,” he says. “But if we can decrease storage time from 1 million to a few hundred years, we’ve come a long way.”Nilsson examines one component at a time, making the process easier to control and track. “If you tried to analyze it all at once, you would get an indication of what happens but you would have no idea why,” he says. “By doing a little at a time you can build a knowledge base, which leads to better methods for processing nuclear waste.”
And although research into separation and recycling of nuclear fuel has been around since the 1950s, only recently has it gained public acceptance in the U.S., where nuclear energy currently accounts for nearly 20 percent of electricity generation. “It’s much more efficient than coal, and if used correctly it doesn’t release anything into the environment,” Nilsson says. “Now we have to convince the public and the politicians that we have the know-how to safely take care of the waste in a cost-efficient manner.”